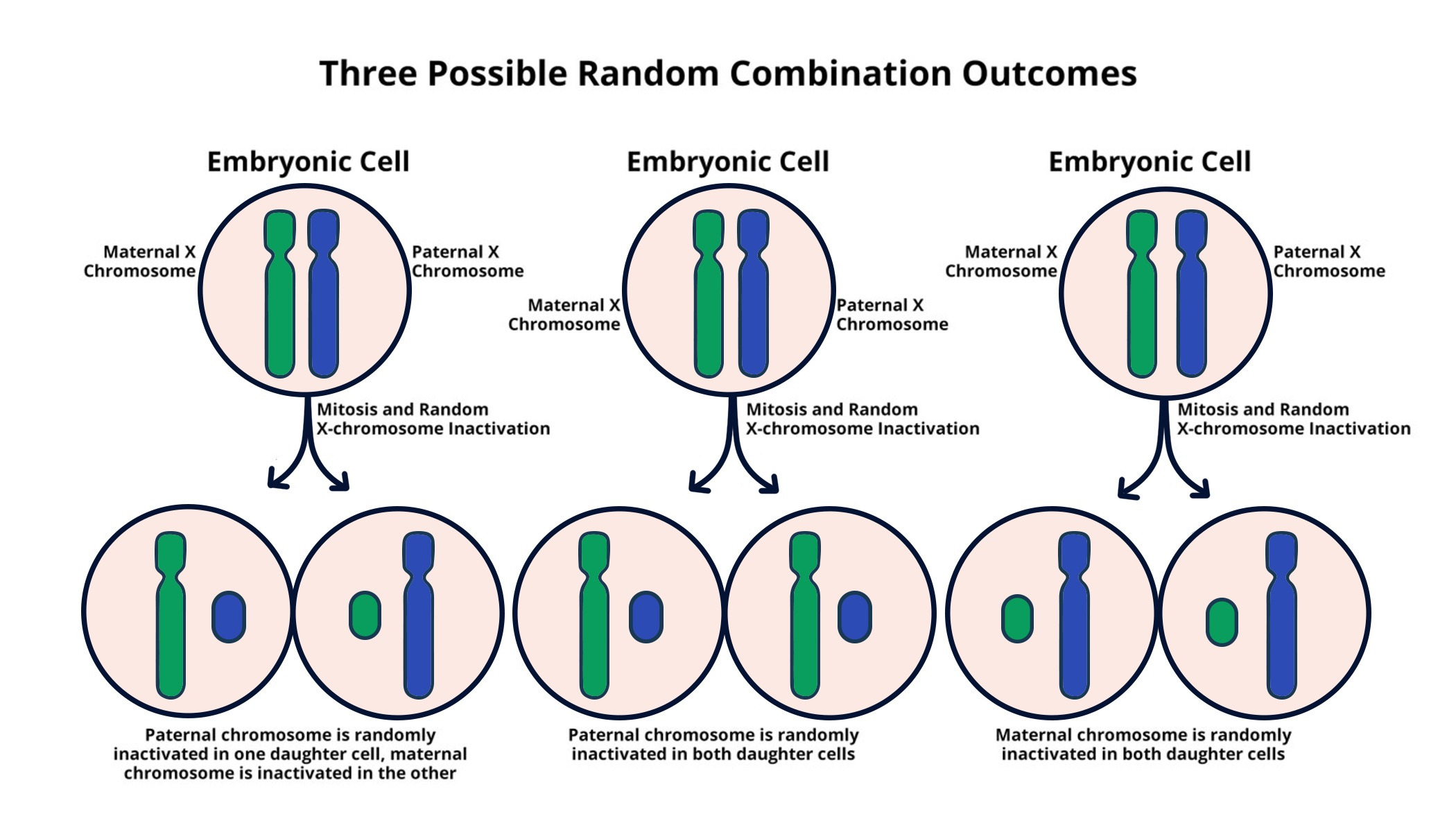
X-chromosome inactivation (XCI) represents a fascinating mechanism by which female mammals regulate gene expression. This chromosomal silencing is crucial for balancing the genetic material from both parents, especially considering that females possess two X chromosomes while males have only one. Understanding XCI has significant implications, particularly for genetic disorders such as Fragile X Syndrome and Rett Syndrome. Recent research led by Jeannie Lee provides insights into how this inactivation process works, shedding light on its potential to unlock therapies for these conditions. By exploring the intricate details of X-chromosome inactivation, scientists are paving the way for groundbreaking treatments that could alleviate the challenges faced by those affected by X-linked diseases.
The process of X-chromosome inactivation, also known as Lyonization, plays a vital role in gene expression balance among female mammals. In this complex biological mechanism, one of the two X chromosomes is silenced to prevent an overload of gene products, a phenomenon that is particularly significant in the context of genetic disorders. Research into chromosomal silencing has unveiled pathways that could lead to therapeutic advancements in conditions like Fragile X and Rett syndromes. Jeannie Lee’s investigations into this topic highlight the potential for innovative treatments derived from understanding how this inactivation is orchestrated within cells. As we further unravel the mysteries surrounding X chromosome biology, we edge closer to effective interventions for those struggling with hereditary genetic conditions.
Understanding X-Chromosome Inactivation and Its Implications
X-chromosome inactivation (XCI) is a critical process that regulates gene expression in females, allowing for a dosage compensation mechanism between sexes. In this fascinating biological phenomenon, one of the two copies of the X chromosome in females is systematically inactivated during early embryonic development. This mechanism ensures that females, who possess two X chromosomes, do not overexpress the genes found on these chromosomes compared to males, who only have one. This silencing process is crucial for normal development and cellular functioning, as disrupted XCI can lead to various genetic disorders.
Recent research by Jeannie T. Lee and her team sheds light on the intricate mechanics of XCI, focusing on the role of an unusual gelatin-like substance that surrounds chromosomes. This substance helps prevent entanglement of chromosomal DNA, enabling precise gene regulation. By understanding the processes underpinning X-chromosome inactivation, researchers hope to devise therapeutic strategies that could potentially ‘un-thaw’ the silenced genes on the X chromosome, opening avenues for curing disorders associated with X-linked mutations such as Fragile X Syndrome and Rett Syndrome.
The Role of Chromosomal Silencing in Genetic Disorders
Chromosomal silencing, particularly of the X chromosome, plays a pivotal role in the manifestation of various genetic disorders. Disorders like Fragile X Syndrome, characterized by intellectual disabilities, are caused by mutations within genes on the X chromosome. The mechanism of XCI often means that these mutations can go unchecked, while the healthy counterparts remain inactive. This poses questions about how to reactivate these silenced genes without disrupting the function of other genes that are crucial for normal cell function.
Jeannie Lee’s research indicates that by manipulating the properties of the chromosomal environment, specifically through the ‘Jell-O’ substance surrounding the chromosomes, it may be possible to restore function to problematic genes. In her quest, Lee aims to take advantage of the findings relative to chromosomal silencing to develop therapies that can selectively activate beneficial genes while leaving unaffected genes intact, thereby minimizing unintended effects typically associated with gene therapy. This innovative approach brings hope for individuals affected by severe genetic disorders and highlights the necessity of continued exploration into the mechanics of chromosomal regulation.
The potential implications of this research are profound. As scientists gain deeper insights into chromosomal silencing mechanisms, they may uncover new methods to treat a variety of genetic disorders that were once deemed untreatable. Such breakthroughs not only promise to alter the landscape of genetic medicine but also raise ethical considerations regarding gene editing and manipulation, urging a balanced approach to employing such powerful technologies.
The Impact of Jeannie Lee’s Research on Fragile X and Rett Syndromes
Jeannie T. Lee’s innovative research into X-chromosome inactivation presents a promising frontier for treating disabling genetic conditions such as Fragile X Syndrome and Rett Syndrome. Fragile X Syndrome is linked to a mutation in the FMR1 gene on the X chromosome, which relies heavily on the intricate balance of gene expression facilitated by XCI. By restoring normal expression of the FMR1 gene through her findings, there is potential to mitigate or even reverse the cognitive impairments associated with this syndrome.
Similarly, Rett Syndrome, primarily affecting females, is caused by mutations in the MECP2 gene, also residing on the X chromosome. Leveraging the mechanisms revealed through Lee’s investigation, there is hope that therapies can be designed to reactivate the silenced gene, allowing for improved neurological function. As her team prepares to move into clinical trials, the medical community and patients alike are eagerly anticipating tangible results that could transform lives. The research is not only a step towards alleviating the challenges posed by these conditions but also serves as a testament to the potential of basic biological research yielding practical applications in medicine.
Decoding the Mechanism of XIST RNA in Chromosomal Dynamics
At the center of the X-inactivation process lies the XIST RNA molecule, a critical player in the initiation of chromosomal silencing. XIST, produced from the X chromosome that is to be inactivated, coats the chromosome and instigates a cascade of modifications within the surrounding chromatin structure. This engagement alters the biophysical properties of the chromosomal environment, making it conducive for silencing. Understanding how XIST interacts with the ‘Jell-O-like’ substance around the chromosome provides essential insights into the cellular dynamics that determine gene expression.
Research from Lee’s lab illustrates the complex interplay between XIST and the surrounding chromatin, akin to a tug of war where XIST ultimately gains the upper hand, leading to the inactivation of the chromosome. By unraveling these dynamics, scientists are not only constructing a comprehensive understanding of XCI but are also paving the way for innovative therapies targeting genetic disorders linked with X-linked genes. The ability to manipulate XIST function opens new pathways for developing treatments that could one day revolutionize the management of diseases such as Fragile X and Rett syndromes.
Navigating the Future of Gene Therapy in X-linked Disorders
The future of gene therapy, particularly for X-linked disorders, is intricately tied to the mechanistic insights gained from research by Jeannie T. Lee. With the groundwork established through understanding X-chromosome inactivation and the roles that various biochemical agents play, the prospect of effective treatments becomes increasingly tangible. The challenge now is to translate these basic scientific discoveries into viable clinical interventions that can selectively activate silenced genes without inadvertently causing harm to healthy genes.
Advancements in gene therapy technologies, informed by Lee’s findings on XCI, hold immense potential to revolutionize treatment approaches for conditions like Fragile X and Rett Syndromes. The ultimate goal of such therapies is to provide effective, supportive care that not only addresses the symptoms of these disorders but also targets their genetic roots. As research continues to evolve, it is crucial to remain mindful of ethical implications and to prioritize safety in the development of any new therapeutic protocols.
The Broader Implications of Chromosomal Research
The exploration of X-chromosome inactivation extends beyond specific diseases, inviting broader discussions around genetic disorders and their management. By decoding the fundamental processes involving chromosomal regulation, researchers contribute to a deeper understanding of genetic diversity, evolution, and the intricate ways genes interact within cellular landscapes. The implications of this research stretch into fields such as developmental biology, medicine, and genetics, highlighting the importance of interdisciplinary collaboration to tackle these complex issues.
Furthermore, the research by Jeannie Lee illustrates the importance of basic scientific inquiry as the foundation for applications that can lead to transformative treatments. It underscores how breakthroughs in one area, such as XCI, can illuminate pathways to address various health challenges faced by individuals with genetic disorders. Continued support for this line of research is crucial, as it could unlock future innovations that reshape therapeutic landscapes across a multitude of diseases.
Future Directions in X-linked Genetic Disorder Research
As researchers like Jeannie T. Lee push the frontiers of knowledge regarding X-chromosome inactivation, the potential future directions for research into X-linked genetic disorders become abundantly clear. The upcoming focus will likely include unraveling various factors affecting the efficacy of XCI and determining how these processes can be manipulated for therapeutic outcomes. Understanding the molecular underpinnings and cellular machinery involved in XCI will be instrumental in developing targeted therapies for conditions like Fragile X Syndrome and Rett Syndrome.
Moreover, the exploration of gene therapy strategies that leverage insights from Lee’s work may usher in an era of personalized medicine for individuals impacted by genetic disorders. The ability to tailor treatments that specifically reactivate beneficial genes while sparing healthy ones will define the next generation of interventions, enhancing both safety and efficacy. As research continues to evolve, the commitment to understanding the complexities of human genetics remains vital.
Challenges and Ethical Considerations in Gene Reactivation Research
While the promise of reactivating silenced genes offers hope for treating debilitating genetic disorders, it also presents significant challenges and ethical considerations that must be addressed. The complexity of the human genome and the nuanced mechanisms of gene regulation complicate the potential for unintended consequences when attempting to manipulate chromosomal functions. Researchers must tread carefully to ensure that therapies do not inadvertently disrupt normal gene expressions or lead to new health issues.
Ethical questions surrounding genetic manipulation, particularly in light of advancements in gene therapy, call for rigorous discussion and oversight. The potential for misuse or unforeseen repercussions emphasizes the need for clear ethical guidelines to govern such research. As we aim to harness the capabilities of gene therapy in a responsible manner, creating frameworks that emphasize transparency and patient safety will be paramount in fostering public trust in these emerging technologies.
The Impact of Continued Funding on Genetic Research
Funding remains an essential element in advancing genetic research and understanding complex biological processes. The support, exemplified by the National Institutes of Health funding Jeannie T. Lee’s groundbreaking research, facilitates critical investigations into X-chromosome inactivation and its implications for genetic disorders. As researchers build upon foundational studies, ongoing financial support is vital for fostering innovation and enabling the translation of findings into clinical applications that can improve patient outcomes.
Moreover, securing sustainable funding allows for the cultivation of diverse collaborations among scientists, medical professionals, and stakeholders. By pooling resources and expertise, the scientific community can navigate the challenges associated with research in genetic disorders more effectively. Ultimately, sustained investment in this field not only enhances our understanding of genetics but also propels the development of practical therapeutic solutions that bear the potential to transform the lives of individuals affected by genetic conditions.
Frequently Asked Questions
What is X-chromosome inactivation and why does it occur in females?
X-chromosome inactivation is a biological process where one of the two X chromosomes in female mammals is silenced to prevent overexpression of X-linked genes. This ensures that females have a similar dosage of X-linked gene products as males, who have only one X chromosome.
How does X-chromosome inactivation relate to Fragile X Syndrome?
Fragile X Syndrome is caused by mutations in the FMR1 gene located on the X chromosome. Due to X-chromosome inactivation, if the affected X chromosome is inactivated, the healthy copy of the gene may remain locked away, leading to the symptoms associated with this genetic disorder.
What role does Jeannie Lee’s research play in understanding X-chromosome inactivation?
Jeannie Lee’s research has been pivotal in uncovering the mechanisms of X-chromosome inactivation. Her lab’s studies explore how chromosomal silencing occurs, significantly contributing to potential treatments for diseases like Fragile X Syndrome and Rett Syndrome.
What are the potential implications of improving X-chromosome inactivation therapies?
Improving therapies related to X-chromosome inactivation could lead to breakthroughs for genetic disorders such as Fragile X and Rett Syndrome. Reactivating the healthy gene on the inactive X chromosome could provide a cure with minimal side effects.
What is the significance of the ‘Jell-O-like substance’ mentioned in relation to X-chromosome inactivation?
The ‘Jell-O-like substance’ refers to the chromatin structure that encapsulates chromosomes, facilitating their organization and regulating gene expression. In X-chromosome inactivation, this substance becomes crucial as it helps in the silencing process orchestrated by molecules like Xist.
Can males benefit from research on X-chromosome inactivation?
Yes, males can benefit from research on X-chromosome inactivation. While they have only one X chromosome, mechanisms similar to chromosomal silencing can affect gene function when mutations are present, such as those causing Fragile X Syndrome.
What challenges remain in the research of X-chromosome inactivation and its related genetic disorders?
Challenges include understanding why freeing inactivated X chromosomes can restore function to mutated genes without impacting healthy genes. More research is necessary to fully grasp the regulatory mechanisms involved and to develop effective clinical applications.
| Key Point | Description |
|---|---|
| X-Chromosome Challenge | Females have two X chromosomes while males have one, leading to the need for inactivation of one X chromosome in females. |
| Role of Xist | A gene on the X chromosome produces Xist RNA, which interacts with a gelatinous substance to initiate inactivation. |
| Mechanics of Inactivation | Xist modifies the properties of the surrounding substance, allowing molecules to access and inactivate the X chromosome. |
| Clinical Implications | Reactivation of inactivated X chromosomes could potentially cure genetic disorders like Fragile X Syndrome and Rett Syndrome. |
| Future Research | Studies are underway to optimize reactivation methods and conduct clinical trials for potential therapies. |
Summary
X-chromosome inactivation is a crucial biological process that ensures females, who possess two X chromosomes, do not overexpress X-linked genes compared to males. Research led by Jeannie T. Lee has provided significant insights into how this inactivation occurs, highlighting the role of the Xist RNA and a gelatinous substance surrounding chromosomes. By understanding and potentially reactivating inactivated X chromosomes, researchers hope to find new treatments for various genetic disorders, positioning this area of study at the forefront of genetic medicine.